Staining of precious metal-plated surfaces has become a critical concern.
 Charles Leech Jr.We must implement drying processes other than CFC displacement. The CFC displacement method failed to remove water from the plated surfaces. It simply floated the rinse water from the surface. Now we must meet the challenge of drying water directly from the plated surface. While staining is a difficult problem, there are some viable solutions.
Charles Leech Jr.We must implement drying processes other than CFC displacement. The CFC displacement method failed to remove water from the plated surfaces. It simply floated the rinse water from the surface. Now we must meet the challenge of drying water directly from the plated surface. While staining is a difficult problem, there are some viable solutions.
Altos Engineering, Inc., was faced with this issue while developing a chemical-free drying process for several electroplating firms that provide 0.9999 gold-plated components to manufacturers of military and commercial semiconductors.
Editor’s Note: This article was originally written in 1994 but remains relevant even today.
The Nature of the Plating Stain
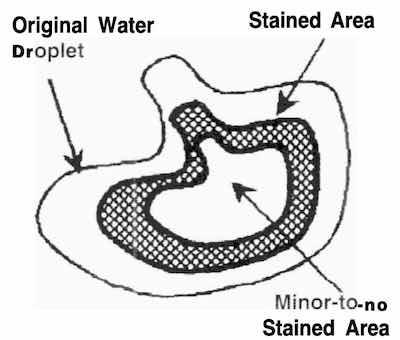 Fig. 1: Formation of a plating stain.While it is very helpful to identify the contaminants that make up the visual defect, their identity is required to solve the staining problem. Potassium cyanide salts cause the bulk of the multicolor stains on gold plating.
Fig. 1: Formation of a plating stain.While it is very helpful to identify the contaminants that make up the visual defect, their identity is required to solve the staining problem. Potassium cyanide salts cause the bulk of the multicolor stains on gold plating.
Stains on other plated surfaces are most likely plating salts as well. It was routinely thought, however, that these salts were removed from the surfaces by the rinsing process. We have evidence that this is not always true.
Previously, the salts were not deposited on the surface when the rinse water was evaporated from the surface.
How Plating Stains Are Formed
Typically, plating stains are seen as irregular-shaped “hollow” blemishes (Fig. 1). The most likely cause is a concentration of the staining salts during drying of rinse water.
Droplets form on wetted areas as the plated items are removed from the rinsewater. If the rinsewater contains dissolved plating salts and/or other water-soluble contaminants, staining is likely to occur. As the rinsewater dries from the surface, the volume of the original water droplet is reduced through evaporation.
As the volume of the droplet decreases, the concentration of contaminants becomes so great that the rinsewater can no longer hold all of it in suspension. At this point, contaminants drop out of the rinsewater and form the stained area. The salts from the rinse water are deposited on the plated surface until no significant amount is present. Reduced staining is seen in the center area, which accounts for the “hollow” stain pattern.
More Effective Post-Plating Processes are Required
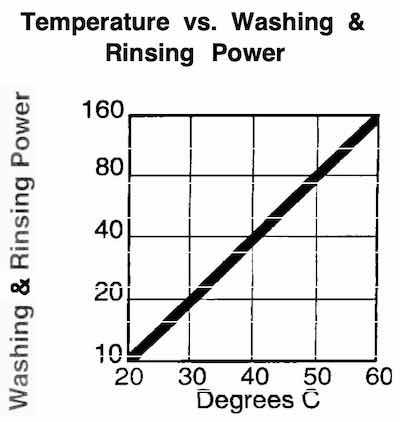 Fig. 2: Temperature vs. solution effectiveness.Three post-plating processes should be examined, to reduce or eliminate plating stains. The first is aqueous-based, between the plating bath and the rinse processes.
Fig. 2: Temperature vs. solution effectiveness.Three post-plating processes should be examined, to reduce or eliminate plating stains. The first is aqueous-based, between the plating bath and the rinse processes.
The second is the plating rinse process, and the third consideration is the method of non-CFC drying. All these should be properly set up, or plating staining will continue.
Cleaning and Rinsing Processes
Adding a simple, aqueous-based cleaning process can be extremely effective in capturing plating contaminants before they can be transferred to the final plating rinse bath. Testing has shown that most commercial aqueous cleaning solutions are not well-suited for removing plating contaminants. The semiconductor industry has been instrumental in driving the development of solutions that are highly effective in removing and capturing plating contaminants. Much of this work was done by Altos Engineering, Inc., as a working member of the JEDEC ad hoc “Marking Improvement Committee.”
Wash and rinse bath temperatures are also very important for effective rinsing of plating residues. As a rule of thumb, rinsing becomes twice as effective as the temperature of a bath is increased by 10 °C. As illustrated in Fig.2, this is a very significant factor.
Agitation is another important factor that can improve the efficiency of postulating washing and rinsing processes. As in the cleaning process, fresh material must be moved across the contaminated surface to remove water-soluble contaminants. The fluid at the interface of the plated surface and the washing and rinsing solutions will become saturated and ineffective very quickly. Fresh, unsaturated solutions must be brought to the contaminated surface on an almost-continuous basis. The agitation method used for effective plating is not always the most effective method for removing plating bath contaminants.
Rinsing Process Considerations
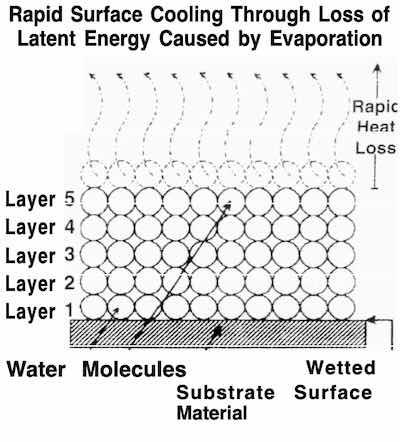 Fig. 3: The physics of vaporization of water.The first consideration should be the quality of the rinse water. The water used by electroplating ranges from city-supplied drinking water to high-purity, deionized water. In between, there is distilled and reverse osmosis-treated water. We have found that deionized water is far more effective in removing plating salts and contaminants from plated surfaces. Deionized water, at a conductivity level of 18 megohms, is most effective in rendering plating salts soluble.
Fig. 3: The physics of vaporization of water.The first consideration should be the quality of the rinse water. The water used by electroplating ranges from city-supplied drinking water to high-purity, deionized water. In between, there is distilled and reverse osmosis-treated water. We have found that deionized water is far more effective in removing plating salts and contaminants from plated surfaces. Deionized water, at a conductivity level of 18 megohms, is most effective in rendering plating salts soluble.
Rinsewater collects high levels of plating contaminants very rapidly. Plating baths that employ a reverse cascade are most effective in removing soluble salts. Frequent replacement of rinse water is critical for effective rinsing.
Drying Processes
There are several industry-accepted methods of removing water from plated surfaces. These include CFC and other water displacement techniques. Hot air knives, convection ovens, alcohol absorption, and centrifugal dryers are commonly used for rugged, non-critical plated objects.
Recently, another non-CFC alternative drying process has been developed. It effectively removes rinse water from such critical articles as those with blind holes, those in intimate contact with each other, those most likely to have plating stains, or those that are delicate. This is thermal/vacuum drying. It was developed for components used in semiconductor and other electronic devices. This is a patented technique that employs a vacuum to reduce the boiling point of the rinse water. Moisture rapidly evaporates from surfaces, cavities, blind holes, and surfaces in contact with one another.
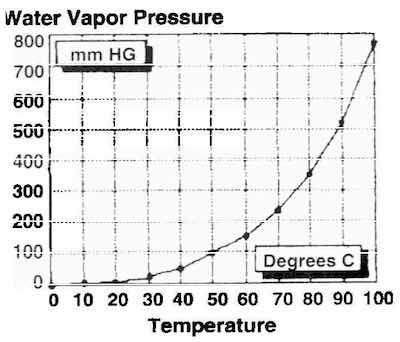 Fig. 4: Vapor pressure of water vs. temperature.Rapid removal of rinse water greatly reduces the incidence of plating stains by removing the water and some salts before they can be deposited on the plated surface. As an example of the speed of thermal/vacuum drying, 3.2 pounds of water can be removed from 18 pounds of deep-drawn, nickel-plated cans in approximately 10 min.
Fig. 4: Vapor pressure of water vs. temperature.Rapid removal of rinse water greatly reduces the incidence of plating stains by removing the water and some salts before they can be deposited on the plated surface. As an example of the speed of thermal/vacuum drying, 3.2 pounds of water can be removed from 18 pounds of deep-drawn, nickel-plated cans in approximately 10 min.
To better understand the fundamentals of drying of water, one must understand some simple physics that pertain to its vaporization. Water can best be thought of as multiple layers of molecules. Each layer is stacked on top of the lower layer. The uppermost layer must be removed before the underlying layers of molecules can be vaporized (Fig. 3).
Under reduced atmospheric pressure, the layers of water rapidly vaporize. With vaporization comes an almost instantaneous loss of temperature. Each gram of water that is vaporized removes approximately 540 BTUs of energy from the upper layer of water.
Water is a poor conductor of thermal energy. It has a thermal conductivity index of 1.6. (Compare this to 2024 aluminum, with an index of 480.) Vaporization under reduced atmospheric pressure occurs so rapidly that thermal conduction from the substrate is too slow to prevent a rapid drop in the temperature of the upper layers of water. As the rinsewater temperature is reduced, the vapor pressure of the water is dramatically reduced (Fig. 4). This impedes the rapid removal of moisture.
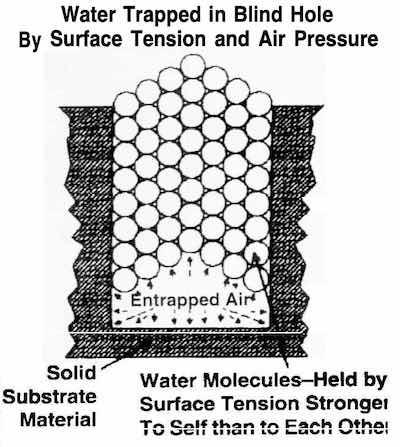 Fig. 5: Expelling water from a blind hole.Without computer-controlled thermal energy to the uppermost surface of the moisture, the vapor pressure of the water will be driven dramatically downward (Fig. 4). The thermal/vacuum drying process applies make-up energy to prevent the drop in vapor pressure.
Fig. 5: Expelling water from a blind hole.Without computer-controlled thermal energy to the uppermost surface of the moisture, the vapor pressure of the water will be driven dramatically downward (Fig. 4). The thermal/vacuum drying process applies make-up energy to prevent the drop in vapor pressure.
Thermal/vacuum drying is effective in removing water from a blind hole, because it reduces the water to a gas (water vapor) which can be almost instantly removed from the drying chamber. This transformation of water into a gas is very effective when dealing with water in crevices and blind holes. The holes are filled with water, but are not always full. Small amounts of air are trapped with the water. When atmospheric pressure is reduced, this entrapped air expands explosively, blowing the water out of the holes. The same reaction is seen when two wet surfaces are in contact with one another.
The time allotted for drying is usually an arbitrary period. The thermal/vacuum system automatically compensates for varying volumes of water to be removed. The system monitors the creation of water vapor, vs. the vacuum level produced by the pump. When a preset level of vacuum is attained, water cannot be present in the process chamber.
Charles S. Leech, Jr., is a graduate of Arizona State University. He has served on the advanced engineering staffs of such firms as Honeywell, Inc., United Technologies, Inc., Texas Instruments, Inc., and Motorola SPD, Inc. He was Director of Engineering for Altos Engineering, Inc. of Glendale, AZ.



































