The subject of hydrogen embrittlement can sometimes confuse end-users, and I often receive a series of questions.
 Frank AltmayerDo all metal finishing procedures cause hydrogen embrittlement?
Frank AltmayerDo all metal finishing procedures cause hydrogen embrittlement?- Does a chemical attack have to take place on the part for hydrogen embrittlement to occur?
- Do all acids cause hydrogen embrittlement?
- How can you passivate heat-treatable grades of stainless steel and avoid this condition?
An understanding of stress, hydrogen effects, and their roles in metal finishing can go a long way toward becoming a valuable troubleshooter in the industry.
In heat-treating operations, we change the crystal structure of steel to yield a harder structure. We can also introduce elements, such as carbon and/or nitrogen, into the steel, forming a dense layer of alloy, commonly referred to as a “case.” Precipitation-hardenable steel alloys, such as 15-5 pH and 17-4 pH stainless steels, date back to only 1959.
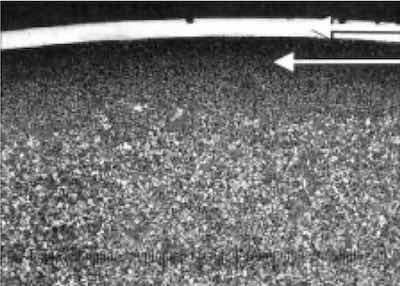 Case-hardened lock shackle. Base metal: steel 12L14. The upper arrow is nickel plating, and the lower arrow is case hardened.These alloys are heat treated and aged at elevated temperature (900°F for 4 hr is one such treatment) to precipitate microscopic “particles” of nickel-aluminum-titanium compounds, which cause internal stresses by distorting the lattice structure and, at the same time, cause an increase in hardness and tensile strength.
Case-hardened lock shackle. Base metal: steel 12L14. The upper arrow is nickel plating, and the lower arrow is case hardened.These alloys are heat treated and aged at elevated temperature (900°F for 4 hr is one such treatment) to precipitate microscopic “particles” of nickel-aluminum-titanium compounds, which cause internal stresses by distorting the lattice structure and, at the same time, cause an increase in hardness and tensile strength.
Anytime a metal is deformed, internal stresses are created. The simple act of deforming steel — such as cold heading, forging, grinding, machining, hammering, blasting, shot peening, and extruding — will impart internal stress on the metal to be finished. The higher the degree of deformation, the higher the hardness. Also, in most cases, the higher the alloying elements in the metal, the higher the internal stress will be.
In general, we must assume that any part that has been heat treated or undergone a significant amount of deformation will be internally stressed.
For ferrous alloys (stainless and otherwise), high internal stress means we must consider the potential of hydrogen embrittlement—more correctly referred to by scientists as hydrogen stress cracking. That is because high internal stresses are areas inside the steel to which hydrogen will diffuse. When hydrogen diffuses to areas of high internal stress, it can destabilize the iron-iron atomic bonds, resulting in the formation of a microscopic crack that, under static tensile loading, will eventually fail the steel at loads far below the maximum design strength.
A previous article addressed the subject of hydrogen stress cracking in some detail but did not address all of your questions, so here goes:
Do all metal finishing procedures cause hydrogen embrittlement?
No, not all metal finishing operations cause or result in hydrogen stress cracking. In many cases, the steel suffers no embrittling effect, even when exposed to hydrogen during metal finishing. This is because only certain types of steel exhibit the problem. Generally, ferrous steels designed or heat treated to tensile strengths above 90,000 psi are sensitive to hydrogen. The higher the design tensile strength of the alloy, the more susceptible the steel is to hydrogen effects, and less exposure to hydrogen will cause the problem.
Case-hardened steel is also sensitive to hydrogen effects. In the case of stainless steels, 200 and 300-series stainless steels are not considered to be affected by exposure to hydrogen. It is best to consider all other stainless alloys as susceptible to the problem.
The metal finishing procedures that cause hydrogen stress cracking are those that allow the susceptible steel to be in a clean condition and to come into contact with hydrogen ions (H+). Common processes that comply with this condition are:
- Cathodic electro cleaning that employs hydrogen gas as a cleaning and deoxidation agent
- Acid immersions, such as pickling or passivation, utilize a solution of water and one or more acids. Acids are chemical compounds that dissociate to form hydrogen ions. There are literature references indicating that the use of an inhibitor can reduce the effects of hydrogen in susceptible steels. Still, these cannot eliminate the problem (and may also cause plating troubles).
- Electroplating can expose the steel to hydrogen, as cathodic polarization during deposition causes the discharge of hydrogen gas in most commonly used electroplating solutions. It is believed that some of this gas dissociates on the clean steel surface and enters the crystal structure of the steel.
Does a chemical attack have to take place on the part for hydrogen embrittlement to occur?
A chemical attack on the part is not required for hydrogen stress cracking to occur. As noted above, cathodic cleaning, which will not attack steel, can cause hydrogen stress cracking in those steels that are sensitive to the hydrogen.
Do all acids cause embrittlement?
All acids should be considered to cause hydrogen exposure to steel (and stainless steel). Whether embrittlement occurs depends on the acid strength, the length of immersion, and the alloy, as noted above.
How can you passivate heat-treatable grades of stainless steel and avoid this condition?
Avoiding exposure to hydrogen can help prevent hydrogen stress cracking. Electrocleaning should be anodic. Avoid acid pickling, if possible, by blasting or other physical preparation.
Exposure to hydrogen in electroplating cannot be eliminated. Because hydrogen diffuses to areas of high triaxial stress, parts that are susceptible to hydrogen damage and have been significantly “worked”—by grinding, machining, etc.—or have been hardened to a high level should be stress-relieved by baking at 375°F before processing for plating or finishing. Otherwise, they may fail by fracturing during plating! Some parts that have been machined and are sensitive to hydrogen can be shot-blasted or peened before plating to eliminate rough surfaces that tend to absorb a higher amount of hydrogen and act as crack-initiation sites.
Because passivation involves immersion of the stainless steel in nitric acid, this operation cannot be modified to avoid hydrogen effects. Other metal finishing processes can avoid the problem, however.
In electropolishing, for example, if the parts go into the process “live” (with some current applied), the parts are anodic, and exposure to hydrogen is not a problem. If parts enter the solution with no applied current, then some exposure to the hydrogen ions in the acid will occur before the power is turned on (see below on how to determine if this is a problem).
Parts can also be mechanically plated instead of electroplated. Keep in mind that even though these steels are sensitive to hydrogen and hydrogen cannot be avoided, we have the means to alleviate the problem after the fact. Baking at 375°F for periods of four to 24 hours, depending on the thickness of the coating, will typically eliminate the hydrogen from the steel and resolve the issue. Note: Four hours is sufficient in the absence of a coating.
If you believe your process does not cause hydrogen stress cracking, you can always process tensile bars— made from the same alloy of steel/ stainless you are processing and machined by ASTM F-519—along with your parts.
After processing, statically load the tensile bars per the specification. If the tensile bars pass the test, your process does not cause hydrogen stress cracking.
Frank Altmayer is a Master Surface Finisher, an AESF Fellow, and the technical education director of the AESF Foundation and NASF. He owned Scientific Control Laboratories from 1986 to 2007 and has over 50 years of experience in the metal finishing industry. He received the AESF Past Presidents Award, the NAMF Award of Special Recognition, the AESF Leadership Award, the AESF Fellowship Award, the Chicago Branch AESF Geldzahler Service Award, and the NASF Award of Special Recognition.



































